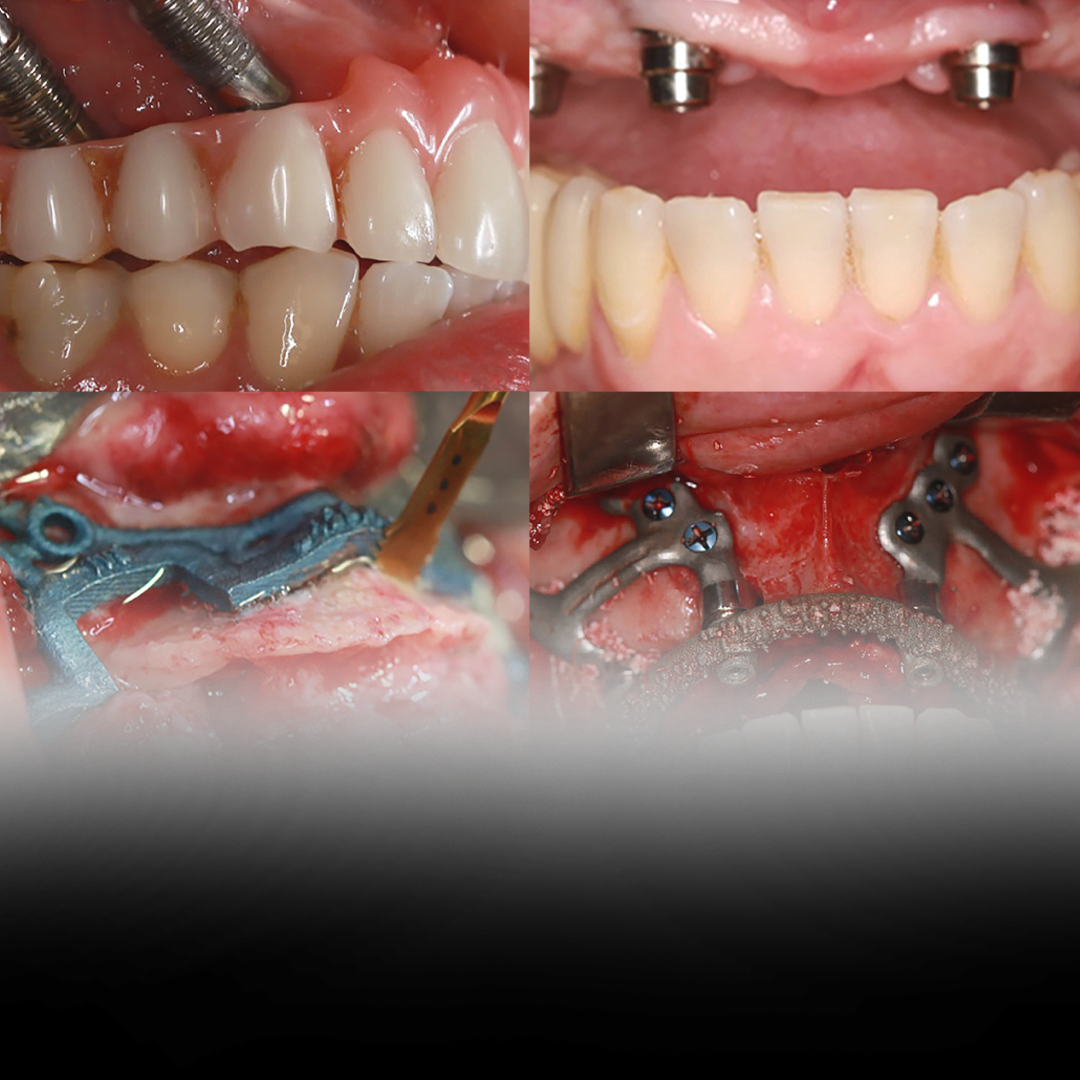
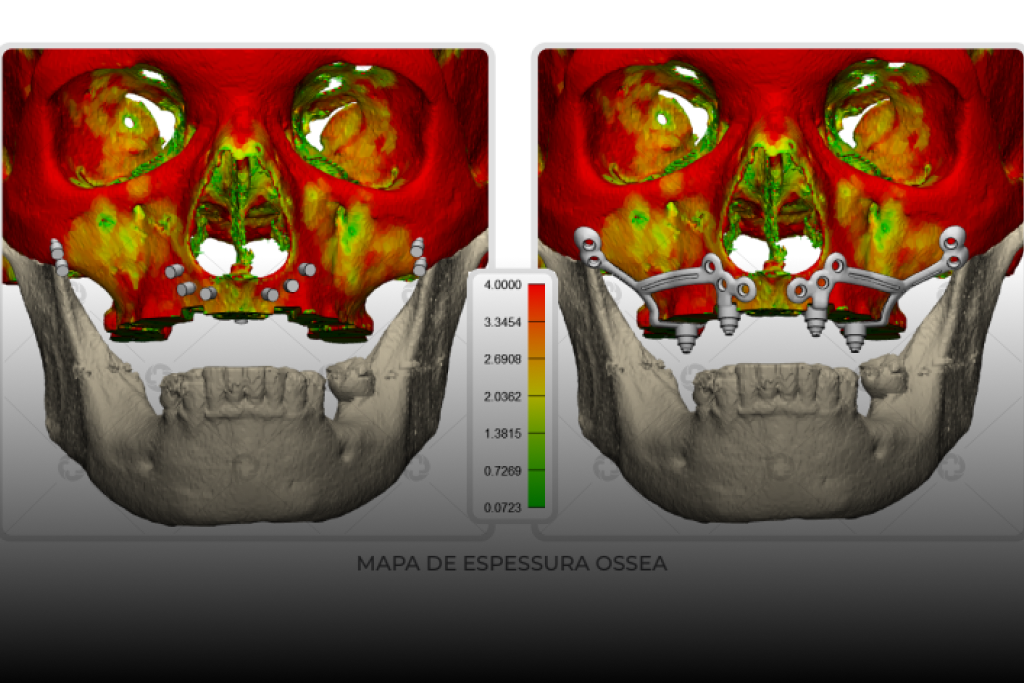
At CPMH, our commitment to providing safe and effective medical device solutions goes beyond the development and manufacturing process itself. We base our policy on a continuous search for improvement in our processes and products, ensuring that our customers always receive the best in innovation and quality.
1. Culture of Continuous Improvement
Continuous improvement is an intrinsic part of our organizational culture. We adopt a philosophy that promotes small, incremental changes that, when added together, result in major advances. We encourage all our employees to identify opportunities for improvement in their daily activities, ensuring that each employee, regardless of their hierarchical level, has a voice and can contribute to the improvement of processes.
2. Investment in Technology and Innovation
At CPMH, we believe that innovation is essential for evolution. That’s why we constantly invest in new technologies and research and development. We follow the latest industry trends and implement advanced technologies such as 3D printing and artificial intelligence, which not only improve the manufacturing of our medical devices, but also ensure greater precision and customization. This focus on innovation allows us to anticipate market needs and offer solutions that meet our customers’ expectations.
3. Training and Qualification
To ensure that our team is always up to date and prepared to face the challenges of the sector, we promote regular training and qualification programs. These programs cover everything from manufacturing techniques to regulatory standards and best quality practices. Investing in the development of our employees is essential to maintaining excellence in our products and services.
4. Customer Feedback
We believe that feedback from our customers is one of the most valuable tools for continuous improvement. We maintain open channels of communication to listen to our users’ opinions and suggestions. We carefully analyze this information to identify areas for improvement and implement them quickly. This interaction not only helps us fine-tune our products, but also strengthens our relationships with our customers.
5. Process Monitoring and Evaluation
To ensure that our processes are constantly evolving, we conduct periodic assessments and monitor performance indicators. We use quality management methodologies that guide our efforts to comply with specific standards and regulations for medical devices. These methods help us identify bottlenecks, optimize resources and ensure operational efficiency.
6. Sustainability and Social Responsibility
Our pursuit of continuous improvement also includes a commitment to sustainability and social responsibility. We seek practices that minimize our environmental impact,
optimizing the use of resources and reducing waste. In addition, we participate in initiatives that promote the health and well-being of the communities in which we operate.
Conclusion
At CPMH, the continuous pursuit of improvement in our processes and products is more than a policy; it is a philosophy that permeates all of our operations. By investing in technology, training and innovation, and by valuing feedback from our customers, we ensure that our medical devices not only meet but exceed market expectations. We are committed to leading with excellence and delivering solutions that truly make a difference in people’s lives.