TMJ
Prosthesis
Customized prosthesis according to the patient’s needs.
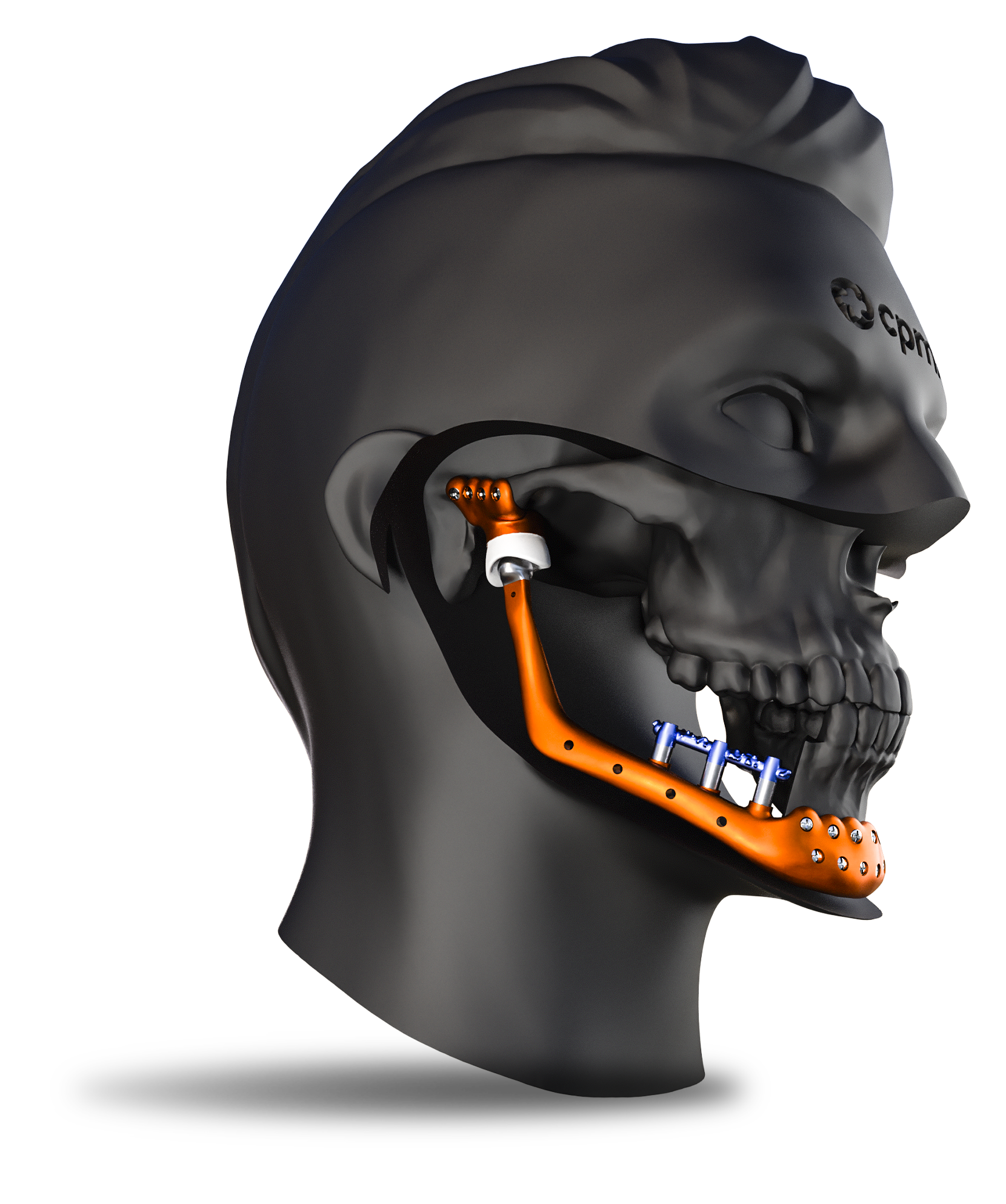

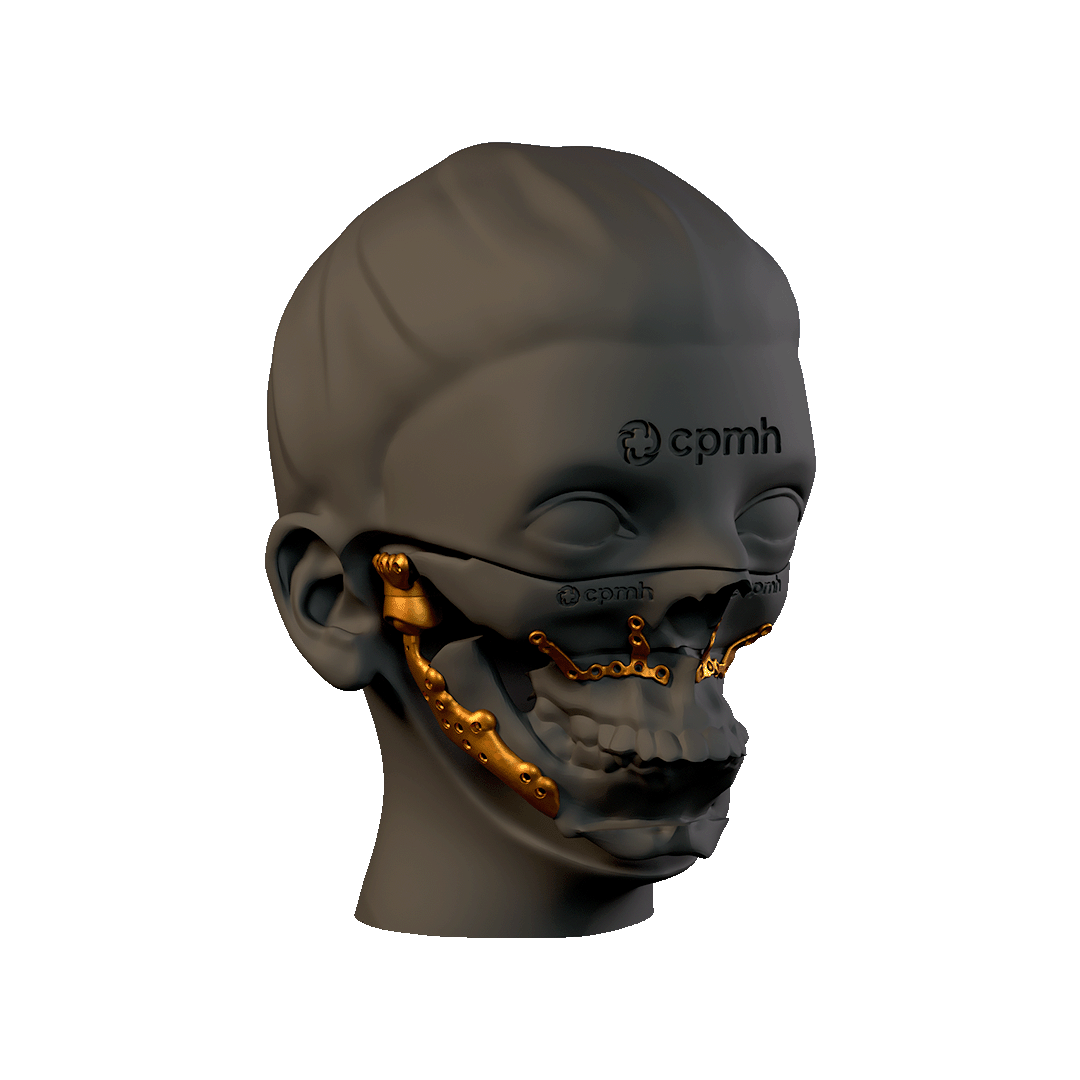

Custom-made
The Custom TMJ prosthesis represents a significant advance in the treatment of temporomandibular joint disorders. With a design adapted to each patient, high-quality materials and a secure fixation system, this prosthesis offers an effective and long-lasting solution for the recovery of jaw functions. The personalized and individualized approach ensures better clinical results and a higher quality of life for patients.
- Molten polyethylene
- Polyethylene with vitamin E
- SLA treatment
- Front and rear stop
- Locking screws, greater stability in fixing
Three-Dimensional Anatomical Analysis
STANDARD TMJ 3DAA
Three-dimensional anatomical CT scan analysis to support the decision on the size of the standard prosthesis to be used, and together with it you will receive the respective drilling and planning devices.
Contraindications
TMJ reconstruction plates should not be used in patients with the following conditions:
- Suspected or present infections at or near the implant site.
- Uncontrollable masticatory muscle hyperfunction.
- Allergy to any of the components of the TMJ reconstruction plates.
- Patient with bone deficiency, quality and/or quantity, a potential situation that may compromise the support/fixation of the components of the TMJ reconstruction plates.
- Incomplete/immature bone development, such as in children.
- Parafunctional habits.
- Implants that present little stability, instability or damage. Disorders that make it difficult to adhere to medical guidelines during the treatment phase.
Installation Procedure
Step 1: Perform surgical access and position the cutting and drilling guides for the mandible and fossa.
Step 2: Use the 1.1 mm drill bit to mark the guide fixing points. Secure the guide with the 1.6 mm screws.
Step 3: Drill the remaining holes for the prosthesis template fixation points.
Note: Pit drilling/cutting guides have 1.6mm metal drill guide walls.
Note: Jaw drilling/cutting guides have 2.0mm metal drill guidance walls
Step 4: Perform osteotomy of the mandible and/or fossa, if necessary, with the piezoelectric instrument or saws.
Step 5: Remove the drilling/cutting guides.
Step 6: Place the interocclusal splints and, optionally, locking screws and steel wire to stabilize the distal segment in the desired position.
Step 7: Position the fossa template and the mandibular component (non-implantable) with 2 screws.
Step 8: Remove the intermaxillary block and perform rotation and translation movements to assess the position and kinetics of the template. If the template and movement are satisfactory, remove the templates.
Step 9: Replace the occlusal splints in position and perform the maxillomandibular block.
Step 10: Position the titanium and polyethylene component of the pit and use the pit positioning instrument to stabilize the pit component.
Step 11: Install the fossa fixation screws with 2.0 mm cortical screws. The choice of screw length is based on clinical-surgical decision. If necessary, use 2.4 mm screws as an emergency.
Step 12: Position the mandibular component and install with the 2.4 mm locking screws.
Step 13: Remove the lock and interocclusal splint
Step 14: Perform rotation and translation movements. If necessary, reposition the prosthesis components.
Step 15: Suture the planes.
Cautions and Warning
- Components of TMJ reconstruction plates are STERILE.
- SINGLE USE product. DO NOT REUSE OR REPROCESS.
- Applying excessive loads increases the risk of deformations, cracks and fractures in the implant.
- Misuse of the implant or improper fixation can lead to loosening of the screws, fractures and/or treatment failure.
- Electrosurgery, radiation exposure and chemotherapy can affect the functionality of TMJ reconstruction plates. Do not use in cases of fractures or signs of fragility in the implant structure.
- Do not use the product if the packaging is damaged or the seal is broken.
- Do not use the product if found damaged.
- Do not use the product after the expiration date.
- Do not use if the model/reference shows any discrepancies.
- Use only compatible products manufactured by CPMH to ensure the safety and effectiveness of the device installation.

Adverse Events
Every surgical procedure presents risks and the possibility of complications. Adverse events may include:
- Loosening, displacement or fracture of components.
- Vascular or neurological impairment due to the surgical procedure.
- Feeling of pain, discomfort or annoyance with the implant.
- Edema, bruising, inflammation, heat sensitivity, infection, exfoliation, perforation or abscess formation, hyperplasia, gingival irregularities, complications associated with anesthesia.
- Mechanical implant failure or exposure.
To download the flyer in PDF, click below
To download technical information in PDF, click below
BLOG & NEWS
Follow our news, articles and events

Maxillary Expansion with Custom Palatal Guide: Precision and Comfort in Orthognathic Surgery
A Revolution in Maxillary Expansion: How Custom Palatal Guides Are Transforming Surgical Outcomes Maxillary expansion following Le Fort I osteotomy remains one of the greatest
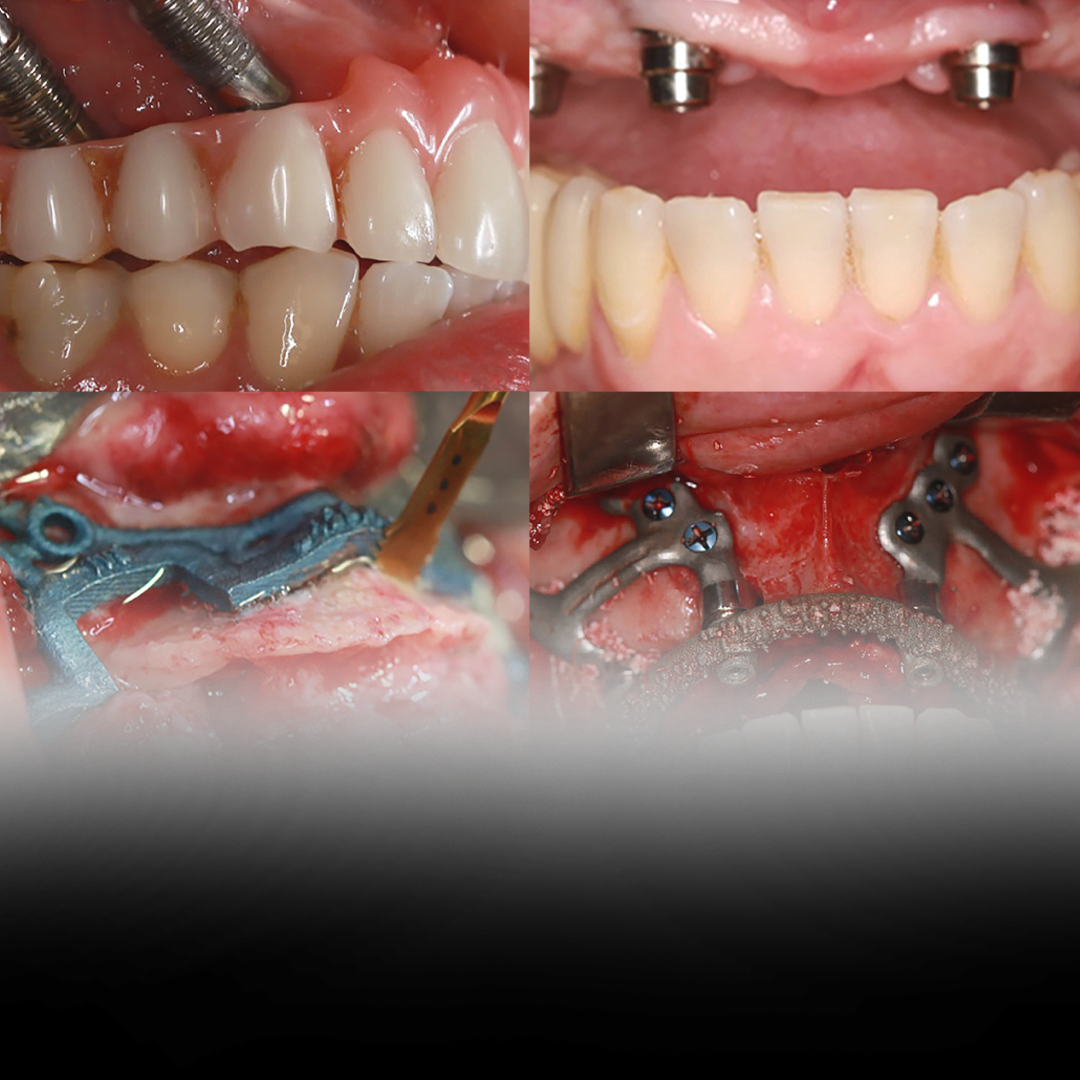
Introduction to Maxillary Rehabilitation and Custom Subperiosteal Implants
Maxillary rehabilitation is an essential field within oral and maxillofacial surgery, especially when dealing with patients who have suffered complications resulting from zygomatic implants. This
Rehabilitation of Atrophic Jaws with Customized Subperiosteal Implants: Advances and Perspectives in Modern Dentistry
Severe maxillary atrophy and edentulism have been recurring challenges in oral and maxillofacial dentistry, requiring increasingly innovative and personalized approaches for effective oral rehabilitation. A