Orthognathic
Personalized
Developed with advanced 3D modeling and additive manufacturing technology, these plates are designed to provide precise fit and stable fixation in orthognathic procedures.
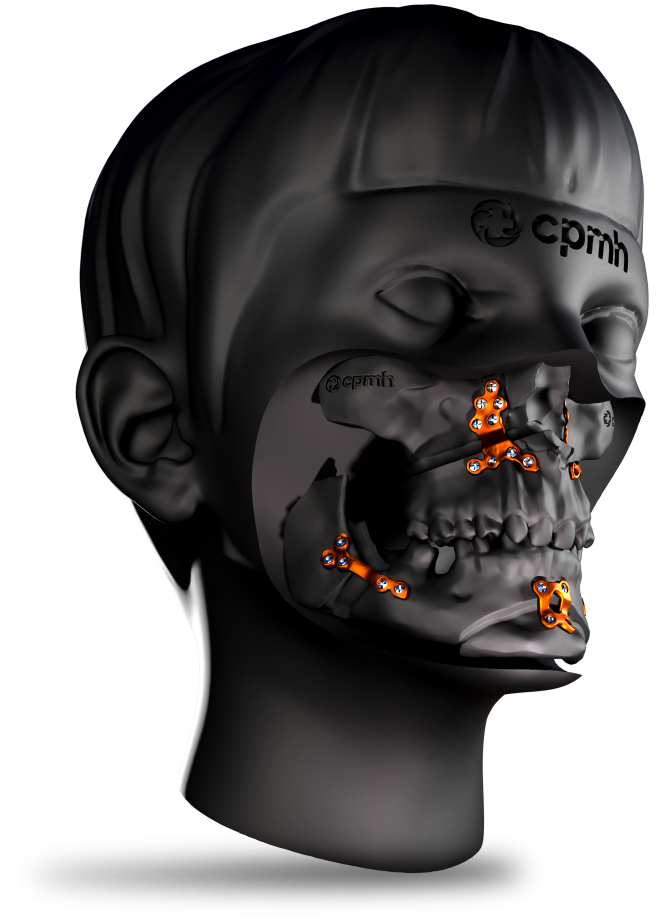


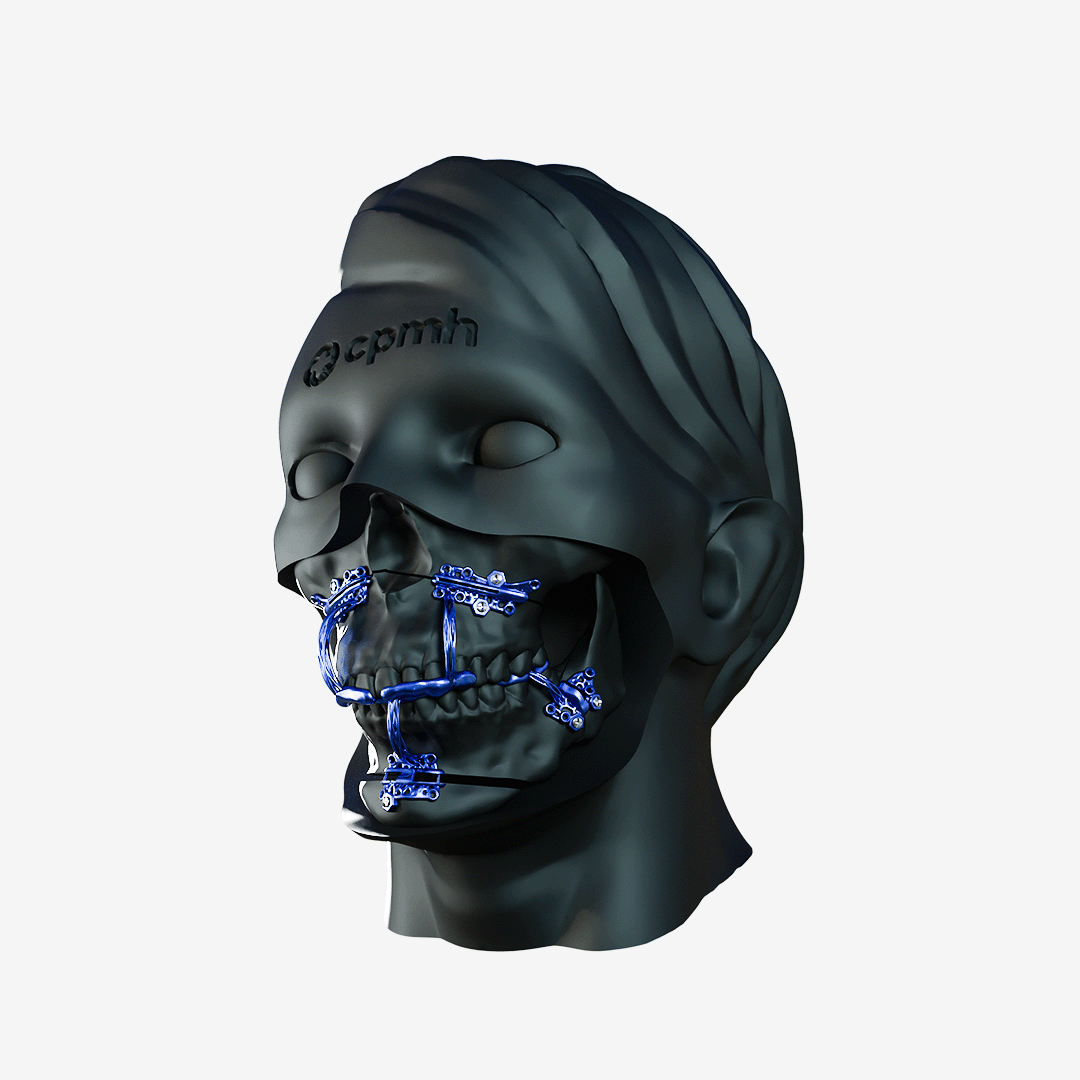
Custom Orthognathic Surgery
Made from high-quality titanium, these plates offer superior strength and proven biocompatibility, ensuring safety and effectiveness during use. Each plate is meticulously tailored to the patient’s specific anatomical needs, promoting surgical precision and optimized results.
Orthognathic
Minimally invasive orthognathic surgery
A revolutionary approach to jaw realignment that combines advanced techniques with quicker recovery times.
Traditionally, orthognathic surgery involved extensive incisions and longer healing periods.
However, the minimally invasive method uses smaller incisions and plates, specialized instruments, and advanced imaging technology to achieve the same results with reduced discomfort and a faster recovery.
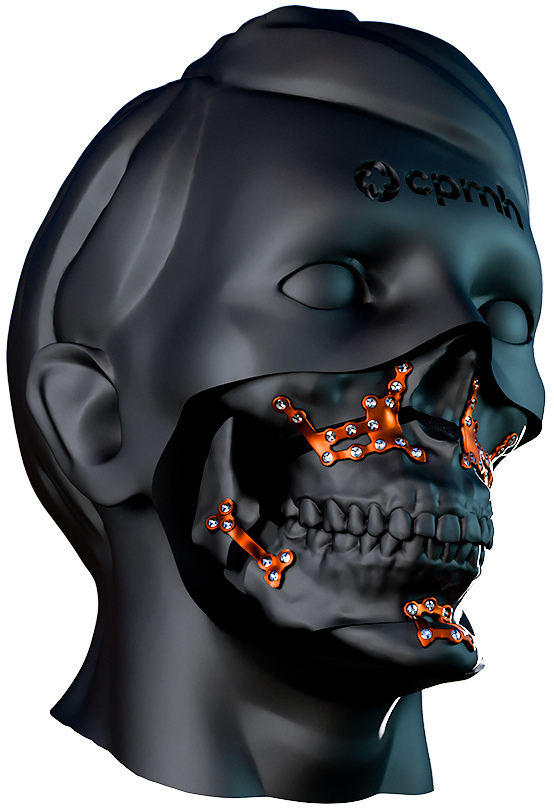
The CPMH Orthognathic Surgery System is a safe, effective and patient-specific solution, manufactured within strict quality control standards.
The plates developed by state-of-the-art 3D modeling and Additive Manufacturing Technology with high-quality Titanium offer excellent resistance and biocompatibility.
The plates are perfectly adapted to the patient’s anatomy for precise and minimally invasive orthognathic surgery.
The system provides surgeons with greater precision due to customized and individualized manufacturing.
The system components include all this.
All components are supplied sterile.
- Maxillary Implants
- Mandible Implants
- Chin Implants
- Screws
- Surgical Guides
- Biomodels

Implants are manufactured to adapt to the patient’s anatomy and specific needs, according to a design specified by the responsible professional.
Contraindications
Osteosynthesis plates should not be used in patients with one or more of the following conditions:
- Infections present in the implantation region;
- Systemic conditions with increased susceptibility to infections, such as immunosuppression and/or conditions that hinder the repair process, such as uncontrolled diabetes, alcoholism, smoking;
- Presence of bone deficiency, qualitative and/or quantitative, a potential situation that may affect the support/fixation of the prosthesis.
- Allergy to any of the components present in the prosthesis;
- Presence of disorders that make it impossible to follow medical advice;
- Incomplete/immature bone development, such as in children for example.
Surgical Procedures
Step 1: Anesthetize the patient
Step 2: Expose the plate attachment area using a
standard surgical approach.
Step 3: Position the surgical guide.
Step 4: Drill the guide screw attachment points
with the appropriately sized drill bit.
Step 5: Remove the surgical guide.
Step 6: Test the adaptation of the osteosynthesis plates on the bone preparation performed. If necessary, make adjustments to the bone to obtain a precise adaptation.
Step 7: Fix the osteosynthesis plates through the most anterior perforations. Any perforations not present should be performed using the 1.5 mm drill bit.
Step 8: Install the screws alternately, without tightening them completely. After positioning all the screws, apply the final torque to tighten the screws.
Note: Screw lengths are provided in the report accompanying the osteosynthesis plates for the orthognathic surgery and must be strictly followed.
Step 9: Suture the incision site.
Cautions and Warning
- The components are provided STERILE.
- Single-use product. DO NOT REUSE OR REPROCESS.
- Applying excessive loads increases the risk of deformation, cracks, and fractures in the implant.
- Improper use of the implant or improper fixation may lead to screw loosening, fractures, and/or treatment failure.
- Electrosurgery, radiation exposure, and chemotherapy may affect the functionality of osteosynthesis plates.
- Do not use in case of fractures or signs of fragility in the structure of the implant.
- Do not use the product if the packaging is damaged or if the seal is broken.
- Do not use the product if it is damaged.
- Do not use the product past the expiration date.
- Do not use if the model/reference presents discrepancies.
- Use only compatible products manufactured by CPMH to ensure the safety and efficacy of the device installation.
Adverse Events
Every surgical procedure carries risks and the possibility of complications. Some common risks associated with any surgical procedure include:
- Infection, bleeding, paresthesia, anesthetic risk, among others.
- Loosening, displacement, or fracture of the components.
- Vascular or neurological impairment due to the surgical act.
- Sensation of pain, discomfort, or unease.
- Edema, hematomas, inflammation, infection, peeling, abscess formation, hyperplasia, gum irregularities, complications associated with anesthesia, implant failure, or exposure.

To download the flyer in PDF, click below
To download technical information in PDF, click below
BLOG & NEWS
Follow our news, articles and events

Maxillary Expansion with Custom Palatal Guide: Precision and Comfort in Orthognathic Surgery
A Revolution in Maxillary Expansion: How Custom Palatal Guides Are Transforming Surgical Outcomes Maxillary expansion following Le Fort I osteotomy remains one of the greatest
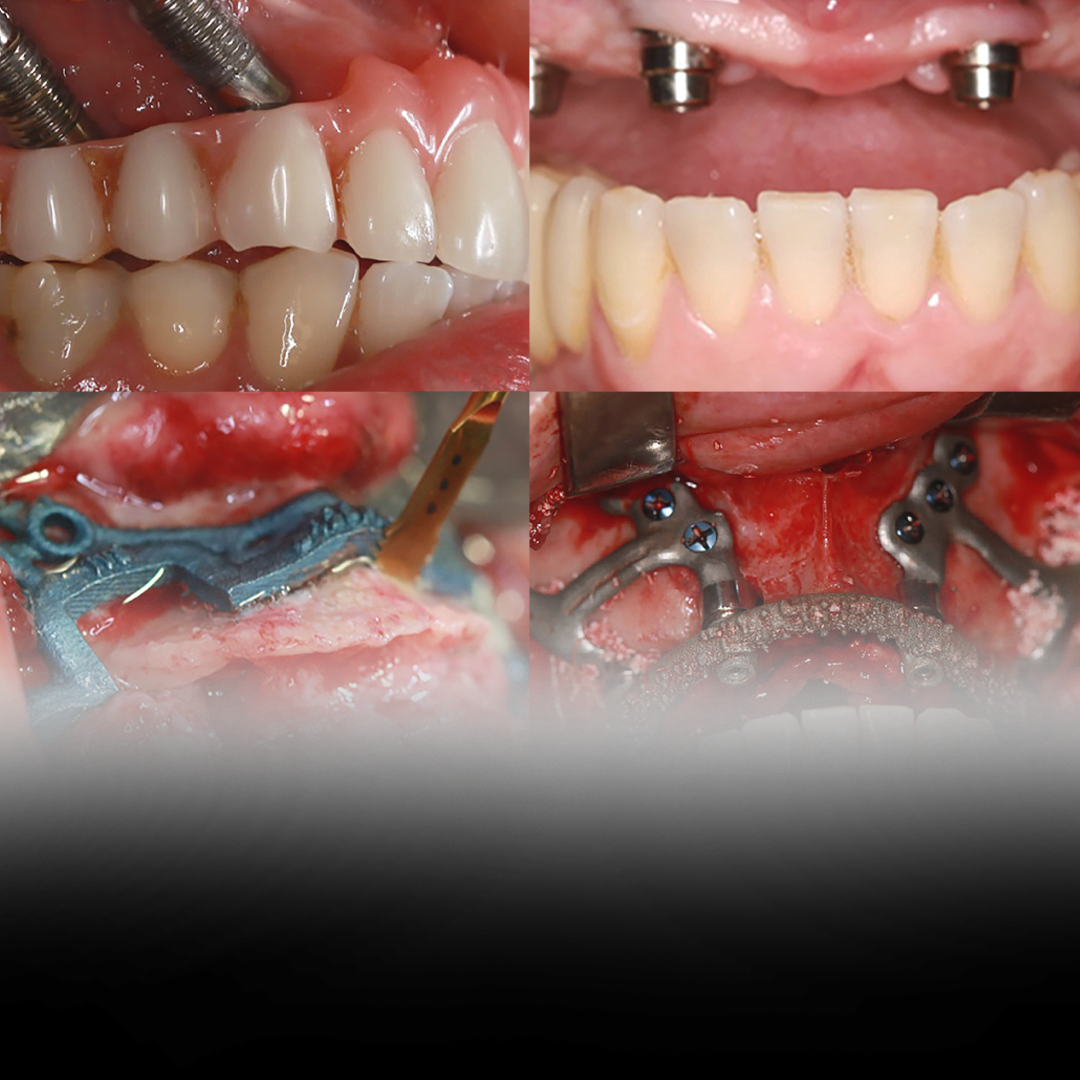
Introduction to Maxillary Rehabilitation and Custom Subperiosteal Implants
Maxillary rehabilitation is an essential field within oral and maxillofacial surgery, especially when dealing with patients who have suffered complications resulting from zygomatic implants. This
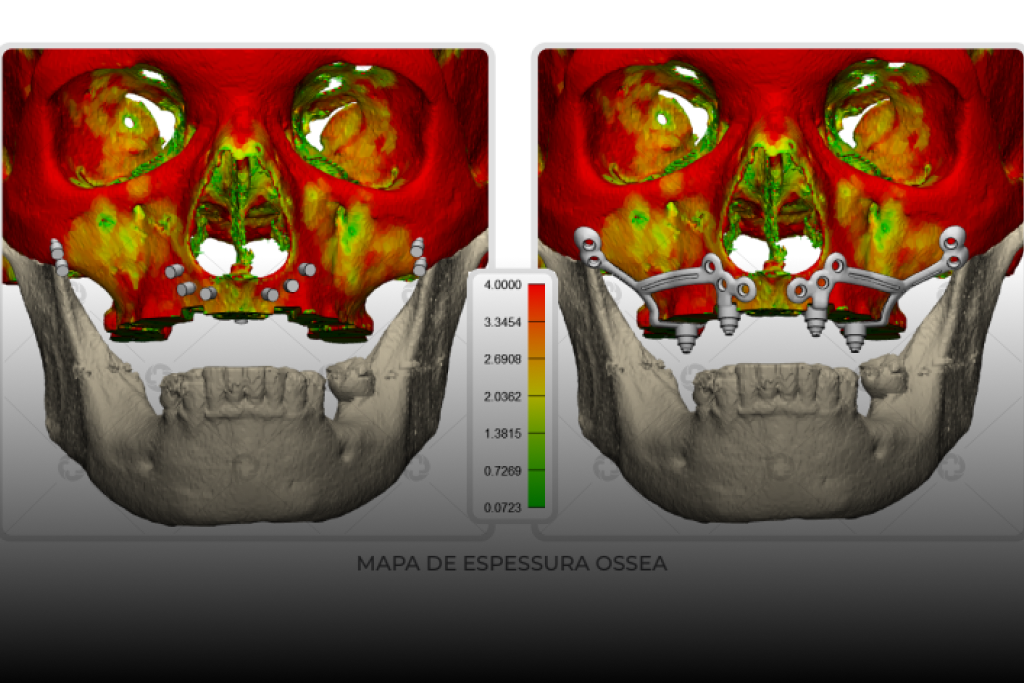
Rehabilitation of Atrophic Jaws with Customized Subperiosteal Implants: Advances and Perspectives in Modern Dentistry
Severe maxillary atrophy and edentulism have been recurring challenges in oral and maxillofacial dentistry, requiring increasingly innovative and personalized approaches for effective oral rehabilitation. A